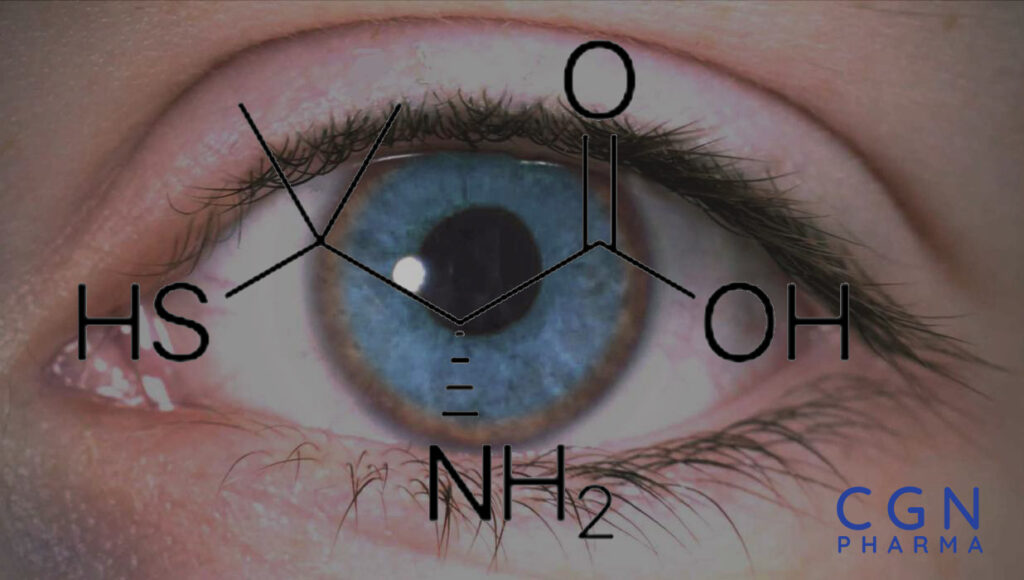
What is D-Penicillamine?
D-penicillamine, a medication derived from the penicillin family, is renowned for its exceptional therapeutic properties and is widely used in the treatment of various medical conditions. Understanding the intricate workings of this drug is crucial for both healthcare professionals and patients alike. To explaining effectively what is D-Penicillamine, we will delve into the fascinating realm of D-penicillamine, exploring its administration process and shedding light on the question, “How does D-penicillamine work?” Join us as we uncover the D-Penicillamine mechanism of action behind this extraordinary medication and its role in managing certain health ailments effectively.
What is the difference between D-penicillamine and L-penicillamine:
D-Penicillamine (C5H11NO2S) exhibits optical isomerism due to its asymmetric carbon atom. Among its isomers, D-Penicillamine adopts the D-configuration of L-cysteine, giving it unique properties. As a thiol compound, D-Penicillamine contains a thiol group (-SH) that sets it apart chemically. It belongs to the chelator class of pharmaceuticals, preferred over L-Penicillamine due to its non-toxic nature. Although it is an α-amino acid metabolite of penicillin, D-Penicillamine lacks antibiotic properties. Its distinctive molecular structure and chelating properties contribute to its therapeutic characteristics and distinguish it from other compounds.
D-Penicillamine Treatment in Wilson’s Disease:
D-Penicillamine is approved on Wilson’s Disease treatment by EMA. Wilson’s Disease arises from impaired copper metabolism and the accumulation of copper in the liver due to a mutation in the ATP7B gene, which disrupts copper transportation. Normally, copper is excreted through the bile pathway, but in Wilson’s Disease, this mechanism is impaired, leading to copper buildup in the liver and subsequent oxidative stress, changes in enzyme activity, and hepatocellular damage.
Wilson’s Disease: Clinical Symptoms and Treatment:
Wilson’s Disease can affect various organs, including the liver, brain, kidneys, and eyes. D-Penicillamine plays a crucial role in its treatment by reducing copper accumulation and preventing toxic effects. It controls the disease’s progression by facilitating the elimination of excess copper through the bile pathway, protecting the liver and reducing copper buildup in the brain to mitigate neurological symptoms. Furthermore, D-Penicillamine prevents copper accumulation in other tissues, safeguarding multiple organs. Extensive clinical studies support its effectiveness, making D-Penicillamine treatment option that can significantly enhance patients’ quality of life and prognosis.
D-Penicillamine Indications:
D-Penicillamine’s therapeutic potential extends beyond Wilson’s Disease. D-Penicillamine is approved for the treatment of conditions such as rheumatoid arthritis, cystinuria, and scleroderma. Additionally, D-Penicillamine is sometimes used off-label for lead toxicity, copper, mercury, and arsenic poisoning, as well as primary biliary cirrhosis. These off-label uses, based on clinical judgment and individual patient needs, offer alternative avenues for utilizing D-Penicillamine’s therapeutic benefits. Depending on the specific condition being treated, the appropriate D-penicillamine dose is determined to optimize its effectiveness and minimize potential side effects. Understanding the D-penicillamine mechanism of action sheds light on its remarkable impact. By reducing copper accumulation, mitigating toxic effects, and preserving the health of vital organs, D-Penicillamine emerges as a powerful weapon in unraveling the enigma of Wilson’s Disease and other related disorders, holding the promise of improved patient outcomes.
D-Penicillamine Side Effects:
While D-penicillamine has proven effective in treating various medical conditions, it’s important to be aware of potential side effects. Common side effects include rash, loss of taste, decreased appetite, vomiting, diarrhea, bleeding, fever, mouth ulcers, and changes in blood cell counts. However, it’s crucial to note that D-penicillamine can also have serious adverse effects such as aplastic anemia, agranulocytosis, thrombocytopenia, Goodpasture’s syndrome, and myasthenia gravis. Close monitoring of symptoms and immediate reporting of any concerns to healthcare professionals are essential when using D-penicillamine. Understanding the risks and benefits helps make informed decisions about its use in managing specific health conditions.
Penicillamine is typically produced in doses of D-Penicillamine 125 mg, D-Penicillamine 250 mg, and D-Penicillamine 300 mg. However, upon request, it can also be contract manufactured in higher or lower dosages such as D-Penicillamine 500 mg. You can buy D-Penicillamine from all around the World in sufficient conditions. D-Penicillamine price can vary depending on the dosage and current factors. To obtain accurate and up-to-date information about Penicillamine pricing, please contact us!
D-Penicillamine Buy OnlineReferences:
WALSHE JM: Penicillamine, a new oral therapy for Wilson’s disease. Am J Med. 1956 Oct;21(4):487-95.
Walshe JM: The story of penicillamine: a difficult birth. Mov Disord. 2003 Aug;18(8):853-9.
Gong Y, Frederiksen SL, Gluud C: D-penicillamine for primary biliary cirrhosis. Cochrane Database Syst Rev. 2004 Oct 18;(4):CD004789.
Suarez-Almazor ME, Spooner C, Belseck E: Penicillamine for rheumatoid arthritis. Cochrane Database Syst Rev. 2000;(2):CD001460.
Munro R, Capell HA: Penicillamine. Br J Rheumatol. 1997 Jan;36(1):104-9.
National Center for Biotechnology Information. PubChem Compound Summary for CID 5852, Penicillamine. https://pubchem.ncbi.nlm.nih.gov/compound/Penicillamine.
Clements PJ, Furst DE, Wong WK, et al (1999) High dose versus low dose D-penicillamine in early diffuse systemic sclerosis. Arthritis Rheumatism 42:1194–203
Dusek P, Roos PM, Litwin T, Schneider SA, Flaten TP, Aaseth J. The neurotoxicity of iron, copper and manganese in Parkinson’s and Wilson’s diseases. J Trace Elem Med Biol. 2015 Jul. 31:193-203.
Rodriguez-Castro KI, Hevia-Urrutia FJ, Sturniolo GC. Wilson’s disease: A review of what we have learned. World J Hepatol. 2015 Dec 18. 7(29):2859-70.
Schilsky ML. Wilson disease: current status and the future. Biochimie. 2009 Oct. 91(10):1278-81.
Kieffer DA, Medici V. Wilson disease: at the crossroads between genetics and epigenetics-A review of the evidence. Liver Res. 2017 Sep. 1(2):121-30.
Bowcock AM, Farrer LA, Hebert JM, et al. Eight closely linked loci place the Wilson disease locus within 13q14-q21. Am J Hum Genet. 1988 Nov. 43(5):664-74.
Stapelbroek JM, Bollen CW, van Amstel JK, et al. The H1069Q mutation in ATP7B is associated with late and neurologic presentation in Wilson disease: results of a meta-analysis. J Hepatol. 2004 Nov. 41(5):758-63.
Stuehler B, Reichert J, Stremmel W, Schaefer M. Analysis of the human homologue of the canine copper toxicosis gene MURR1 in Wilson disease patients. J Mol Med (Berl). 2004 Sep. 82(9):629-34.
Merle U, Schaefer M, Ferenci P, Stremmel W. Clinical presentation, diagnosis and long-term outcome of Wilson’s disease: a cohort study. Gut. 2007 Jan. 56(1):115-20.
Manolaki N, Nikolopoulou G, Daikos GL, et al. Wilson disease in children: analysis of 57 cases. J Pediatr Gastroenterol Nutr. 2009 Jan. 48(1):72-7.
Roberts EA, Schilsky ML; American Association for Study of Liver Diseases (AASLD). Diagnosis and treatment of Wilson disease: an update. Hepatology. 2008 Jun. 47(6):2089-111.
Yoshitoshi EY, Takada Y, Oike F, et al. Long-term outcomes for 32 cases of Wilson’s disease after living-donor liver transplantation. Transplantation. 2009 Jan 27. 87(2):261-7.
Schilsky ML. Wilson disease: diagnosis, treatment, and follow-up. Clin Liver Dis. 2017 Nov. 21(4):755-67.
Li WJ, Chen C, You ZF, Yang RM, Wang XP. Current drug managements of Wilson’s disease: from west to east. Curr Neuropharmacol. 2016. 14(4):322-5.
Walshe JM. Copper: its role in the pathogenesis of liver disease. Semin Liver Dis. 1984 Aug. 4(3):252-63.
Dastych M, Prochazkova D, Pokorny A, Zdrazil L. Copper and zinc in the serum, urine, and hair of patients with Wilson’s disease treated with penicillamine and zinc. Biol Trace Elem Res. 2010 Mar. 133(3):265-9.
Langwinska-Wosko E, Litwin T, Dziezyc K, Czlonkowska A. The sunflower cataract in Wilson’s disease: pathognomonic sign or rare finding?. Acta Neurol Belg. 2016 Sep. 116(3):325-8.
Soni D, Shukla G, Singh S, Goyal V, Behari M. Cardiovascular and sudomotor autonomic dysfunction in Wilson’s disease–limited correlation with clinical severity. Auton Neurosci. 2009 Dec 3. 151(2):154-8.
Dziezyc K, Litwin T, Chabik G, Czlonkowska A. Measurement of urinary copper excretion after 48-h d-penicillamine cessation as a compliance assessment in Wilson’s disease. Funct Neurol. 2015 Oct-Dec. 30(4):264-8.
Tarnacka B, Szeszkowski W, Golebiowski M, Czlonkowska A. Metabolic changes in 37 newly diagnosed Wilson’s disease patients assessed by magnetic resonance spectroscopy. Parkinsonism Relat Disord. 2009 Sep. 15(8):582-6.
Sen S, Felldin M, Steiner C, et al. Albumin dialysis and Molecular Adsorbents Recirculating System (MARS) for acute Wilson’s disease. Liver Transpl. 2002 Oct. 8(10):962-7.
Weiss KH, Thurik F, Gotthardt DN, et al. Efficacy and safety of oral chelators in treatment of patients with Wilson disease. Clin Gastroenterol Hepatol. 2013 Aug. 11(8):1028-1035.e2.
Weiss KH, Gotthardt DN, Klemm D, et al. Zinc monotherapy is not as effective as chelating agents in treatment of Wilson disease. Gastroenterology. 2011 Apr. 140(4):1189-98.e1.
da Costa Mdo D, Spitz M, Bacheschi LA, Leite CC, Lucato LT, Barbosa ER. Wilson’s disease: two treatment modalities. Correlations to pretreatment and posttreatment brain MRI. Neuroradiology. 2009 Oct. 51(10):627-33.
Brewer GJ, Askari F, Dick RB, et al. Treatment of Wilson’s disease with tetrathiomolybdate: V. control of free copper by tetrathiomolybdate and a comparison with trientine. Transl Res. 2009 Aug. 154(2):70-7.
Chaudhry HS, Anilkumar AC. Wilson Disease. StatPearls [Internet]. 2019 Jan 11. 2018:
Gerosa C, Fanni D, Congiu T, et al. Liver pathology in Wilson’s disease: from copper overload to cirrhosis. J Inorg Biochem. 2019 Jan 15. 193:106-11.
Ala A, Borjigin J, Rochwarger A, SchilskyM. Wilson diseasein septuagenarian siblings: raising the bar for diagnosis. Hepatology 2005;41:668–70.
Brewer GJ, Terry CA, Aisen AM, Hill GM. Worsening ofneurologic syndrome in patients with Wilson’s disease withinitial penicillamine therapy. Archives of Neurology 1987;44:490–3.
Brewer GJ, Fink JK, Hedera P. Diagnosis and treatment of Wilson’s disease. Seminars in Neurology 1999;19(3):261–70.
Brewer GJ. Neurologically presenting Wilson’s disease:epidemiology, pathophysiology and treatment. CNS Drugs 2005;19(3):185–92.
Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration,2011.
Liang XL, Yang RM, Wu ZY, Wang N, Li XH, Wang X. A Chinese Guideline on Diagnosis and Treatment of Hepatolenticular Degeneration. Chinese Journal of Neurology 2008;41:566‐569.
Patil M, Sheth KA, Krishnamurthy AC, Devarbhavi H. A review and current perspective on Wilson disease. JOURNAL OF CLINICAL AND EXPERIMENTAL HEPATOLOGY 2013;3:321‐336.
Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. The Nordic Cochrane Centre, The Cochrane Collaboration.Review Manager (RevMan). 5.3.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roberts EA, Schilaky ML. Division Gastroenterologyand Nutrition. A Practice Guideline on Wilson Disease. Hepatology 2003;37:1475–92.
Roberts EA, Schilsky ML. Diagnosis and treatment of Wilson disease: an update. Hepatology 2008;47:2089‐2111.
Schilsky ML. Treatment of Wilson’s disease: What arethe relative roles of penicillamine, trientine, and zinc supplementation?. Current Gastroenterology Reports 2001;3:54–9.
Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpretingresults and drawing conclusions. In: Higgins JPT, GreenS (editors), Cochrane Handbook for Systematic Reviewsof Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration 2011;Available from handbook.cochrane.org.
Starosta‐Rubinstein S, Young AB, Kluin K, Hill G, AisenAM, Gabrielsen T, et al. Clinical assessment of 31 patientswith Wilson’s disease. Correlations with structural changeson magnetic resonance imaging. rchives of Neurology 1987;44:365–70.
Sternlieb I. Perspectives on Wilson’s disease. Hepatology 1990;12:1234–9.
Walshe JM. Wilson’s disease: new oral therapy. Lancet 1956;270:25–6.
Walshe JM, Waldenström E, Sams V, Nordlinder H, Westermark K. Abdominal malignancies in patients with Wilson’s disease. Q J Med 2003;96:657‐662. [PubMed] [Google Scholar]
Wilson SAK. Progressive lenticular degeneration: a familialnervous disease associated with cirrhosis of the liver. Brain 1912;34:295–507.
Yang Renmin. hepatolenticular degeneration [肝豆状核变性]. Book 1995:167, 184, 205, 255..
Renmin Yang, Nan Cheng. Observation on short‐term effect and follow‐up of integrative medicine in treating 198 patients with hepatolenticular degeneration [中西医结合治疗198例肝豆状核变性患者的近期疗效及随访观察]. Chinese journal of integrated traditional and western medicine 2002;22(9):657‐659.
This article is intended for healthcare professionals (e.g., medical doctors, pharmacists, and pharmaceutical traders).